Invisible Risk: Why Dust Control is a Critical Priority in Cleanroom Design for the Pharmaceutical Industry
In the pharmaceutical industry, dust is more than just a hygiene issue; it’s a critical risk to product quality, personnel safety, and regulatory compliance. From weighing and mixing APIs to tableting and capsule filling, tiny particles can be generated at every stage of the process. Failure to rigorously control this dust can have catastrophic consequences—from rejecting entire batches of product to irreversible health damage to operators.
Thus, a truly GMP-compliant cleanroom design involves more than just air cleanliness levels; it also requires a sophisticated, proactive dust control system. This article will delve into the risks posed by pharmaceutical dust and systematically outline the core technologies and strategies used within the industry to mitigate these risks.
What is “pharmaceutical dust”? It’s more than just a cleanliness issue.
Pharmaceutical dust primarily refers to solid particles generated during the production process, suspended in the air, or deposited on equipment surfaces. It primarily consists of:
Active pharmaceutical ingredient (API): This is the most critical component, possessing pharmacological activity.
Excipients: These ingredients, such as starch, lactose, and microcrystalline cellulose, constitute the inactive portion of the drug product.
Mixtures: Mixed particles of API and excipients.
These dusts, especially those from highly active or toxic APIs, present one of the most significant challenges in cleanroom environmental control.
Uncontrolled dust: Three core risks in pharmaceutical production
Product Quality Risk: Cross-Contamination
Cross-contamination occurs when a component of one drug (especially an API) accidentally contaminates another. Even microgram levels of contamination can render a product ineffective, produce toxic side effects, or trigger an allergic reaction in patients. This can not only result in significant financial losses but also severely damage a company’s reputation and patient trust.
Personnel Safety Risks: Occupational Exposure
Many APIs are biologically active, cytotoxic, or allergenic. Chronic inhalation of these dusts, even at low doses, can lead to serious occupational illnesses. Therefore, the industry uses occupational exposure limits (OELs) to define safe concentrations of specific substances in the air. Ensuring that dust concentrations in the work environment are well below the OEL is an unshirkable responsibility of the company.
Regulatory Compliance Risks: GMP Noncompliance
Global pharmaceutical regulatory agencies, such as the US FDA and the EU EMA, have stringent GMP regulations for preventing cross-contamination and protecting operator safety. Any negligence in dust control can result in audit failures, warning letters, or even production suspensions.
The “Pyramid” Strategy for Pharmaceutical Dust Control: A Systematic Approach
Effective dust control doesn’t rely on a single technology; it’s a multi-layered, systematic approach, spanning the source, the environment, and ultimately personnel. It can be divided into three tiers.
Level 1: Containment at the Source – Engineering Control Techniques
The most effective strategy is to capture and control dust the moment it’s generated.
Isolators: This is the highest level of physical containment. Operators, wearing gloves, handle highly active or sterile materials in a completely enclosed environment. This protects both the drug (sterile) and personnel (highly active).
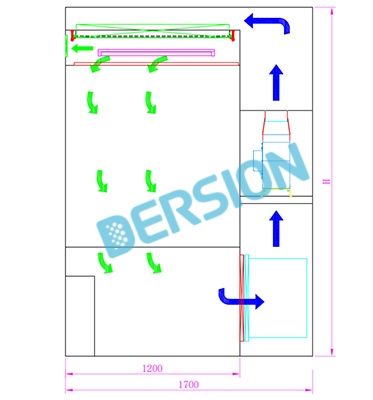
Downflow/Laminar Flow Booths: Dust generated in operating areas (such as weighing and dosing) is forced downward through vertical, unidirectional airflow, where it is captured by filters at the bottom. This provides a safe, localized environment for open or semi-open operations. Local Exhaust Ventilation (LEV): Hoods are installed at specific points around dust-generating equipment, such as tablet presses and mixers, to directly extract generated dust and prevent it from dispersing throughout the room.
Second Level of Control: Environmental Barriers – Cleanroom Facility Design
When dust inevitably escapes from the source, the cleanroom design itself becomes a second and crucial line of defense.
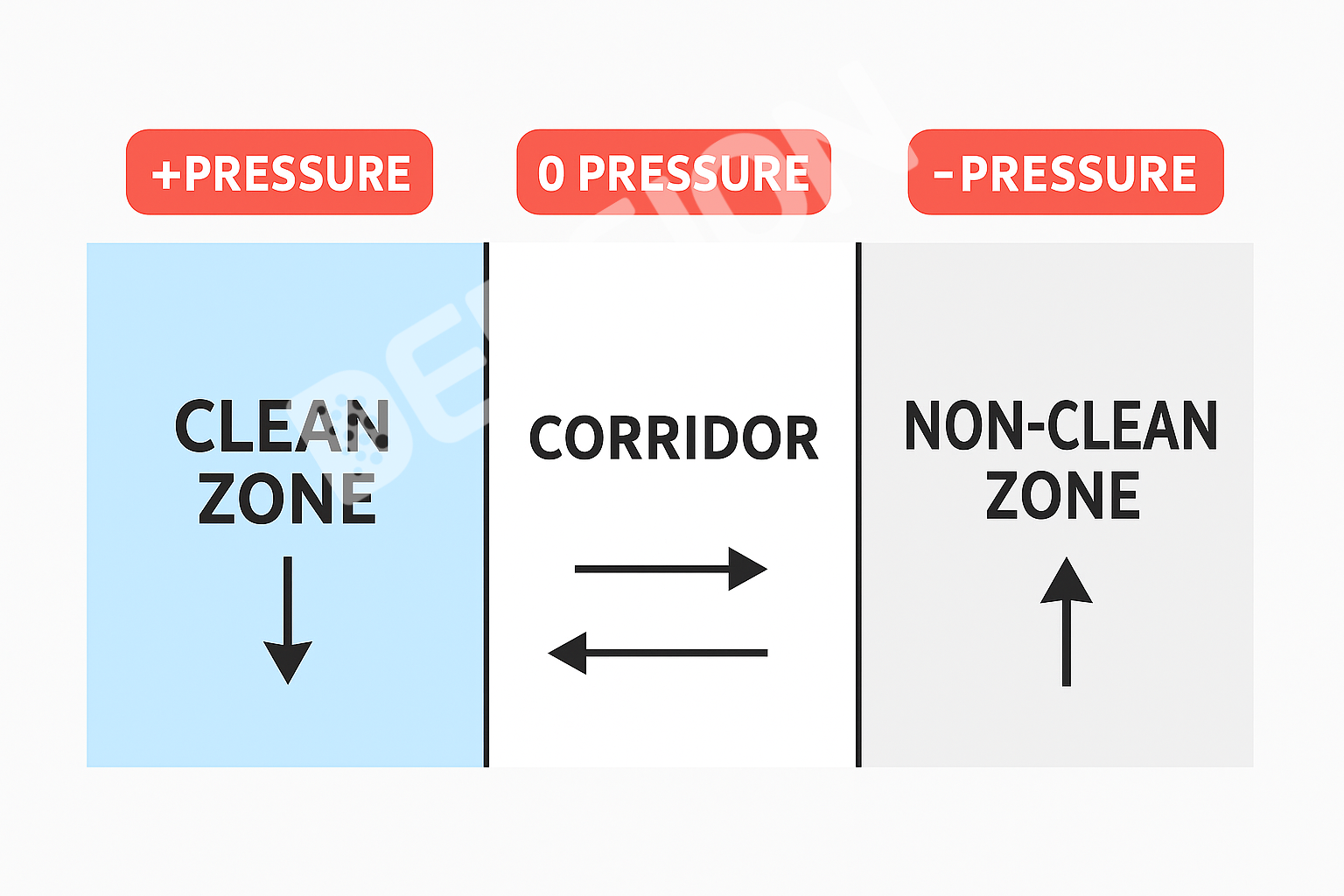
Strict pressure cascades establish stable pressure gradients by precisely controlling the supply and exhaust air volumes between different rooms. For example, high-risk dust-generating operating rooms typically maintain negative pressure to ensure air flows from low-risk areas outside and prevent dust from leaking out. Cleanrooms, on the other hand, maintain relatively positive pressure to prevent air from flowing from the rooms into the corridors.
Optimized HVAC and filtration systems: The HVAC system not only regulates temperature and humidity but also plays a key role in controlling air flow and removing contaminants. HEPA (high-efficiency particulate air) filters are standard. For exhaust systems handling highly reactive dust, safe-change filters (bag-in/bag-out, BIBO) must be used to ensure that maintenance personnel are not exposed to trapped toxic dust when replacing saturated filters.
Smooth, seamless interior surfaces: Cleanroom walls, floors, and ceilings must be constructed of seamless, non-shedding, corrosion-resistant, and easy-to-clean materials (such as epoxy flooring and pre-painted steel wall panels). All joints are rounded to eliminate sanitary dead corners, leaving no place for dust to hide and greatly facilitating cleaning and disinfection.
Level 3 Control: Operating Procedures—SOPs and Personnel Management (Procedural Controls)
The last line of defense is people. Strict operating procedures and well-trained personnel are essential.
Standard Operating Procedures (SOPs): Develop detailed procedures for cleaning, material transfer, personnel entry and exit, and equipment operation.
Personnel Clothing and Training: Employees must receive rigorous training to understand the hazards of dust and master the correct cleanroom clothing and operating procedures.
Cleaning Validation: Validated cleaning methods must be in place to demonstrate that specific cleaning procedures effectively reduce API residues on equipment surfaces to below acceptable levels.
Dersion’s Integrated Solution: Integrating Dust Control into the Cleanroom’s “DNA”
At Dersion, we believe that effective dust control cannot be an isolated feature addition; it must be integrated into the overall cleanroom design concept from the outset of the project.
Risk Assessment First: We collaborate with the client’s EHS and process teams to first assess the OEL values of the materials being handled and the dust generation risks of the process, using these as the fundamental basis for design. System Engineering: We consider pressure gradients, HVAC layout, airflow management, engineered control equipment (such as weigh hoods), and the building envelope as a holistic system, ensuring that each subsystem works together to achieve optimal containment and control.
Compliance Design: All our designs are designed to meet the stringent requirements of global GMPs (such as FDA cGMP and EU GMP Annex 1), ensuring your facility is designed from the ground up to successfully pass future audits and certifications.
FAQ
1.What is the OEL, and why is it important?
The OEL (Occupational Exposure Limit) is a guideline upper limit for the concentration of a hazardous substance in the air below which almost all workers can repetitively perform daily exposures without adverse health effects. It is a key factor in determining the level of dust control equipment (such as isolators or weigh hoods).
2.How to choose an isolator or weigh hood?
The choice depends primarily on the activity and toxicity of the API. For highly active or cytotoxic compounds with very low OELs (e.g., <10 μg/m³), an isolator is often necessary. For low- and medium-risk materials, an efficient weighing hood can provide adequate protection.
3.Is a negative pressure cleanroom the only way to control dust?
Not necessarily, but it is crucial. Negative pressure is a “zone-based” control strategy that prevents dust from dispersing outside the dust-generating room. It must be combined with source control (such as isolators) and high-efficiency air filtration to form a complete control system.
4.How to handle flammable and explosive dust?
For organic auxiliary material dusts such as starch and sugar, the explosion risk must be considered. Cleanroom design must include explosion-proof measures, such as using explosion-proof electrical equipment, increasing exhaust ventilation, installing explosion vents, and employing inert gas shielding.
5.What is the relationship between cleaning validation and cleanroom dust control?
They are closely related. A well-designed cleanroom with smooth surfaces and no blind spots greatly simplifies the difficulty and complexity of cleaning tasks, making it easier to pass cleaning validation. Effective airborne dust control also reduces secondary deposition on equipment surfaces, helping to maintain cleanliness.
Post time: Oct-17-2025