Clean Room Negative Pressure: Definition, Standards & Best Practices
Negative pressure cleanrooms play a critical role in preventing cross-contamination in industries such as medical device manufacturing, pharmaceutical production, biotechnology, and pathogen research. This article breaks down how negative pressure cleanrooms work, key ISO standards, design requirements, and why many international factories choose Dersion’s modular cleanroom systems for high-risk environments.
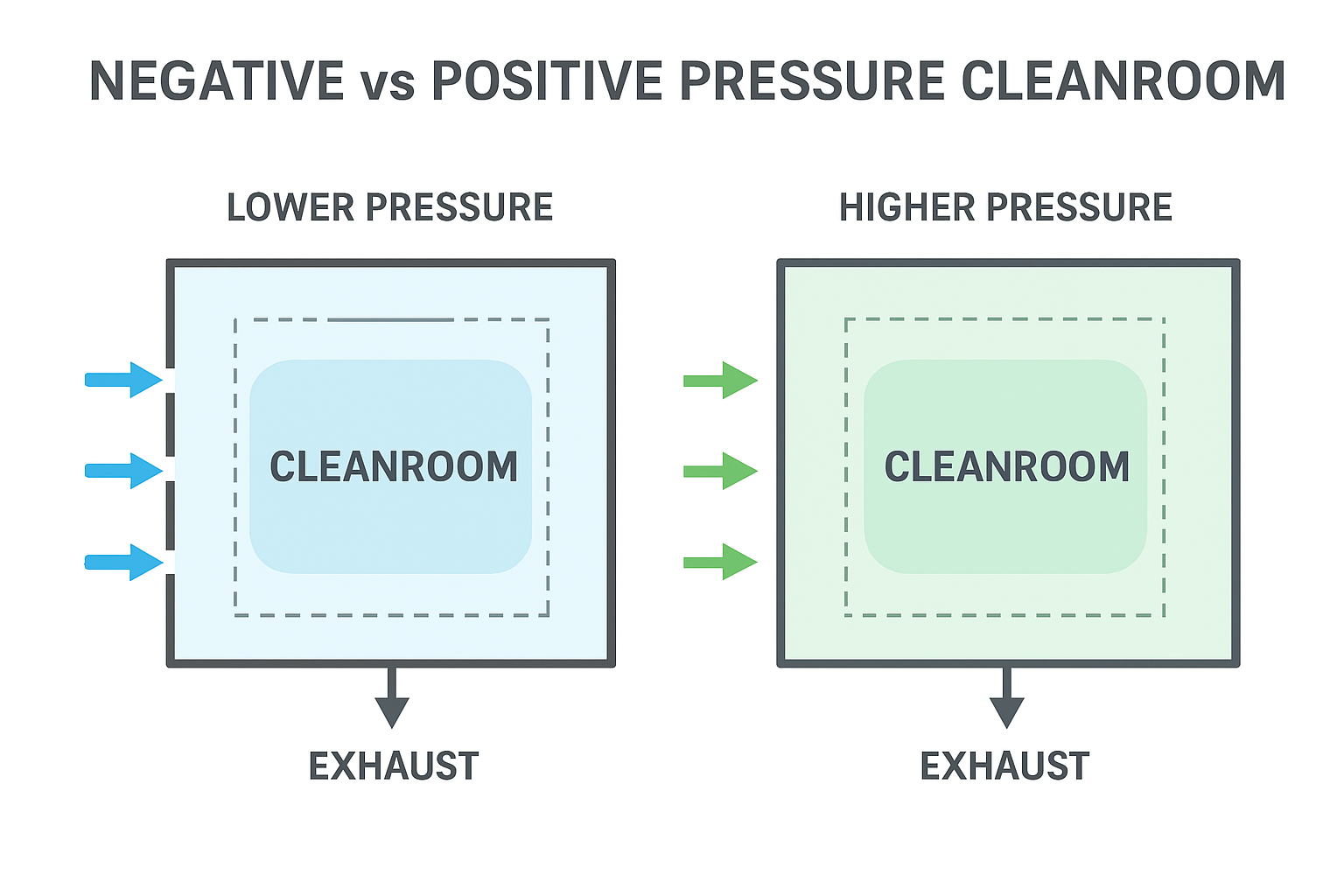
What Is a Negative Pressure Cleanroom?
A negative pressure cleanroom is a controlled environment where the air pressure inside the room is lower than the surrounding areas.
This differential pressure ensures:
1.Air flows into the cleanroom
2.Air does not escape to adjacent spaces
3.Containment of hazardous or contaminated particles
It is designed for rooms where outward leakage must be prevented, such as:
1.Sterile mixing rooms
2.High-risk contamination labs
3.Hazardous drug compounding
4.Biological safety areas
5.Infectious disease diagnostic rooms
Why Negative Pressure Is Needed in Medical & Pharma Facilities
Negative pressure cleanrooms are essential when protecting the external environment is more critical than protecting the internal environment.
Key Benefits
-
Prevents contamination from exiting the room
-
Protects operators and nearby production lines
-
Supports ISO 14644 cleanliness standards
-
Ensures compliance with FDA, GMP, and EU Annex 1 requirements
-
Ideal for processes involving particulates, aerosols, powders, solvents, or biological contaminants
These functions are especially important in:
Medical Device Manufacturing
-
1.Coating & adhesive bonding
-
2.Polymer molding and finishing
-
3.Powder handling
-
4.Device sterilization buffer rooms
Pharmaceutical Production
-
1.APIs and chemical synthesis
-
2.Cytotoxic drug compounding
-
3.Hazardous powder mixing
-
4.Viral vector processing
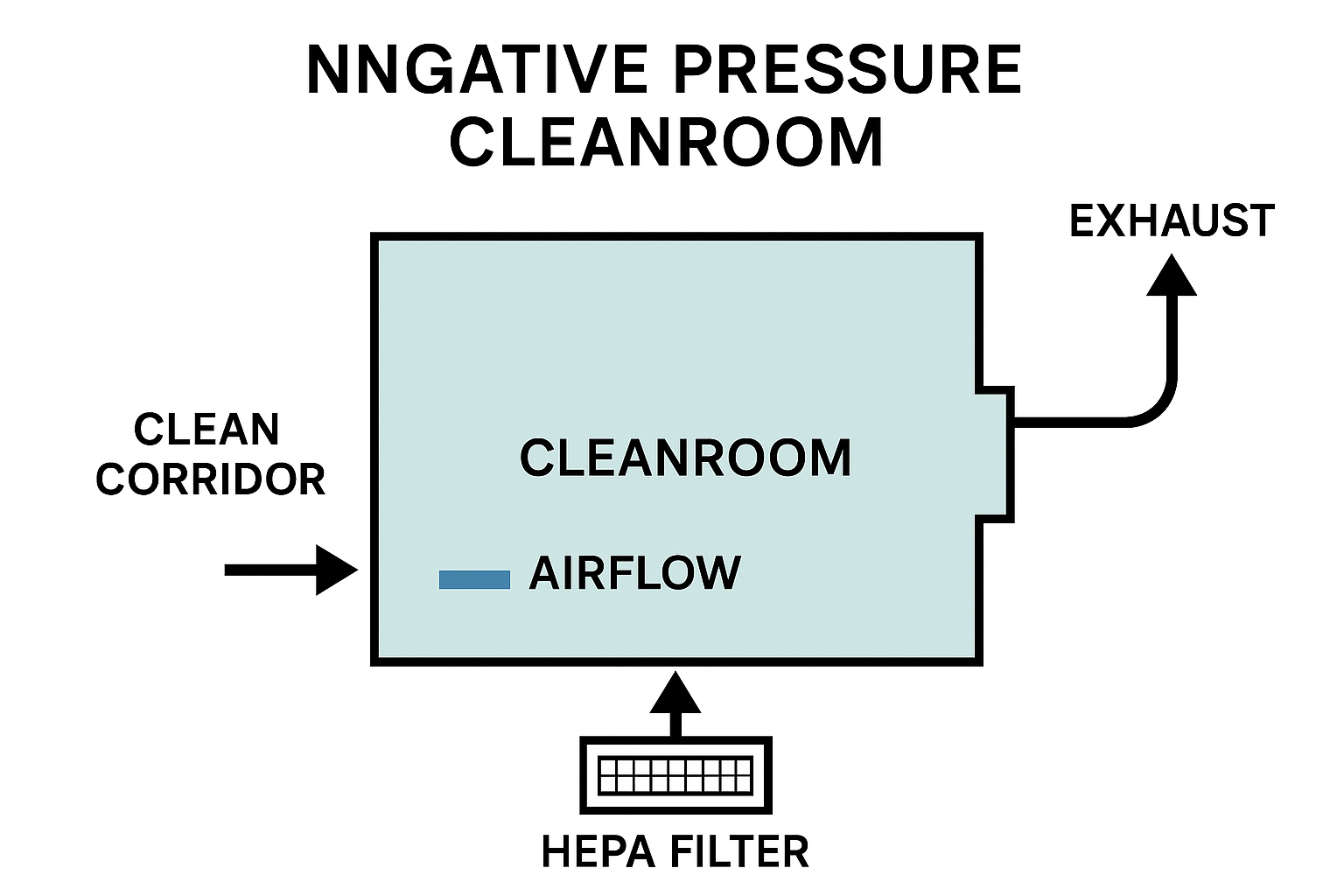
How Negative Pressure Cleanrooms Work (Airflow Concept)
Negative pressure cleanrooms maintain lower internal pressure via:
1. Exhaust-dominant HVAC system
Exhaust > Supply
More air is removed than supplied, pulling air inward from cleaner areas.
2. Controlled airflow paths
Air is designed to flow from:
Clean corridor → Airlock → Negative pressure cleanroom → Exhaust system
3. HEPA/ULPA Filtration
All outgoing air passes through:
-
HEPA H13/H14 filters
-
Or ULPA U15 filters for high-risk applications
4. Pressure differential standards
Typical pressure differences:
-
−15Pa to −30Pa (common medical/pharma standard)
-
≥ −12Pa (minimum stability requirement)
ISO Standards for Negative Pressure Cleanrooms
Negative pressure rooms may be classified under:
ISO 7 Negative Pressure Rooms
Used for medium-risk containment processes:
-
1.Powder filling
-
2.Raw material handling
-
3.Medical coatings
-
4.Polymer grinding
ISO 8 Negative Pressure Rooms
Used for general-risk environments:
-
1.Chemical storage
-
2.General compounding
-
3.Secondary packaging
Design Principles for Negative Pressure Cleanrooms
A compliant negative pressure cleanroom must achieve:
1. Stable Pressure Differential Control
Automated sensors track:
-
1.Pressure levels
-
2.Airflow volume
-
3.Fan speed adjustment
2. Segmented Air Locks
Includes:
-
1.Personnel airlock (PAL)
-
2.Material airlock (MAL)
-
3.Decontamination buffer area
3. High-Efficiency Exhaust System
Must connect with:
-
1.HEPA exhaust hoods
-
2.Chemical-resistant ducts
-
3.Safe discharge pathways
4. Modular Cleanroom Walls & Ceilings
Fire-resistant materials are often required.
Why Choose Dersion Modular Cleanrooms for Negative Pressure Rooms?
Dersion’s negative pressure cleanrooms are engineered for global pharmaceutical and medical device manufacturers who demand:
1.High-precision HVAC balancing
Stable −15Pa to −30Pa differential.
2.Modular expandable structure
Up to 98% reuse rate, ideal for upgrading production lines.
3. 20,000 m² smart factory & German TRUMPF metal processing
Ensures strong structural sealing and long-term durability.
4. One-stop solution: Design → Engineering → Validation
Includes ISO 14644 testing, pressure balancing, and documentation support.
FAQs About Negative Pressure Cleanrooms
1.Is a negative pressure cleanroom safer?
Yes—negative pressure prevents hazardous contaminants from spreading outside the production area.
2.Is ISO 7 or ISO 8 better for negative pressure?
It depends on the risk level:
-
ISO 7 → Medium/high contamination risk
-
ISO 8 → General risk
3.Can negative pressure rooms be modular?
Yes. Dersion’s modular cleanrooms support both negative and positive pressure configurations.
Conclusion
Negative pressure cleanrooms are essential for containing hazardous materials, preventing environmental contamination, and maintaining compliance with global medical and pharmaceutical regulations. With proper ISO classification, pressure control, and advanced HVAC engineering, manufacturers can ensure a safe and compliant production process.
Dersion’s modular negative-pressure solutions offer scalable, validated systems suitable for FDA-regulated and GMP-certified facilities.
Post time: Nov-21-2025