ISO 13485 / GMP / FDA-Compliant Cleanroom Systems
End-to-end cleanroom design, manufacturing,and installation for medical device production and assembly.
- 1.ISO 14644 / ISO 13485 Compliant
-
2.Modular & Fast Deployment
-
3.Global Certifications: CE / UL / ISO
-
4.Designed for FDA & GMP Audits
Key Challenges in Medical Device Cleanroom Projects
Industry Expertise
We have deep experience in delivering ISO/GMP-compliant cleanrooms specifically for Class I–III medical devices.
→ We understand your regulatory challenges, and we build to match them.
Global Project Support
Whether in Asia, North America, or the Middle East, our team provides remote guidance or on-site support for cleanroom installation and training.
→ We’re ready wherever you are.
Turnkey Solutions
We provide end-to-end services: on-site measurement, custom design, engineering, production, trial assembly, shipping, and after-sales support.
→ One-stop service reduces coordination risks and accelerates project timelines.
In-House Manufacturing
Our cleanroom panels, doors, windows, and FFUs are all manufactured in our own ISO-certified facility, ensuring strict quality control, better lead time, and cost-efficiency.
→ You’re dealing directly with the source—no middlemen.
Precision Engineering & Modular Flexibility
Using our proprietary modular system, we deliver flexible and scalable cleanroom spaces that can be expanded, reconfigured, or relocated easily.
→ Future-proof your production environment.
Regulatory Compliance & Validation Support
We ensure compliance with ISO 14644, ISO 13485, FDA CFR 820, and GMP standards, and provide detailed documentation for audits and validation.
→ You get more than a room—you get peace of mind
Medical Device Industry Application Scenarios
Typical Products
- Syringes, infusion sets, blood collection tubes
- Catheters, drainage bags
- Medical masks and protective garments
Cleanroom Focus
- Particle and microbiological control
- High-volume, continuous production stability
Typical Cleanliness Level
- ISO Class 7–8
- Local critical zones: ISO Class 6
Key Cleanroom Areas
- Injection molding rooms
- Assembly and sealing areas
- Pre-sterilization buffer zones
Typical Products
- Orthopedic implants (plates, screws, joints)
- Cardiovascular stents and valves
- Dental implants
Cleanroom Focus
- Ultra-low particle levels
- Control of metal debris and surface contamination
Typical Cleanliness Level
- ISO Class 5–7 (depending on process)
Key Cleanroom Areas
- Post-machining cleaning rooms
- Surface treatment and coating areas
- Sterile packaging rooms
Typical Products
- Diagnostic reagent kits
- Test strips and cartridges
- Microfluidic chips
Cleanroom Focus
- Microbial contamination prevention
- Stable temperature and humidity control
- Batch-to-batch contamination avoidance
Typical Cleanliness Level
- ISO Class 7–8
- Local critical operations: ISO Class 6
Key Cleanroom Areas
- Reagent preparation rooms
- Filling and dispensing zones
- Drying and packaging areas
Typical Products
- Patient monitors and infusion pumps
- Ventilators
- Medical electronic modules
Cleanroom Focus
- Dust and ESD control
- Clean environment for electronic assembly
Typical Cleanliness Level
- ISO Class 7–8
Key Cleanroom Areas
- PCB assembly rooms
- Module assembly zones
- Functional testing and packaging areas
Typical Products
- Sterile surgical instruments
- Disposable sterile kits
- Sterile catheters and dressings
Cleanroom Focus
- High sterility assurance level (SAL)
- Integration with sterilization processes
Typical Cleanliness Level
- ISO Class 5–7
Key Cleanroom Areas
- Sterile assembly rooms
- Sterile packaging areas
- Clearly defined pressure-controlled buffer zones
Application Scope
- Primary and secondary packaging
- Sterile barrier systems (Tyvek, blister packs)
Cleanroom Focus
- Prevention of secondary contamination
- Clear personnel and material flow
Typical Cleanliness Level
- ISO Class 7–8
Application Scope
- Product development and prototyping
- Process validation (IQ, OQ, PQ)
- Small-batch trial production
Cleanroom Focus
- High flexibility and modularity
- Fast deployment and easy reconfiguration
Typical Cleanliness Level
- ISO Class 6–8 (adjustable)


Cleanroom Design Built for Regulatory Approval
Applicable Processes
Medical Device Assembly & Aseptic/Non-aseptic Packaging &Injection Molding, Welding, Testing, Labeling



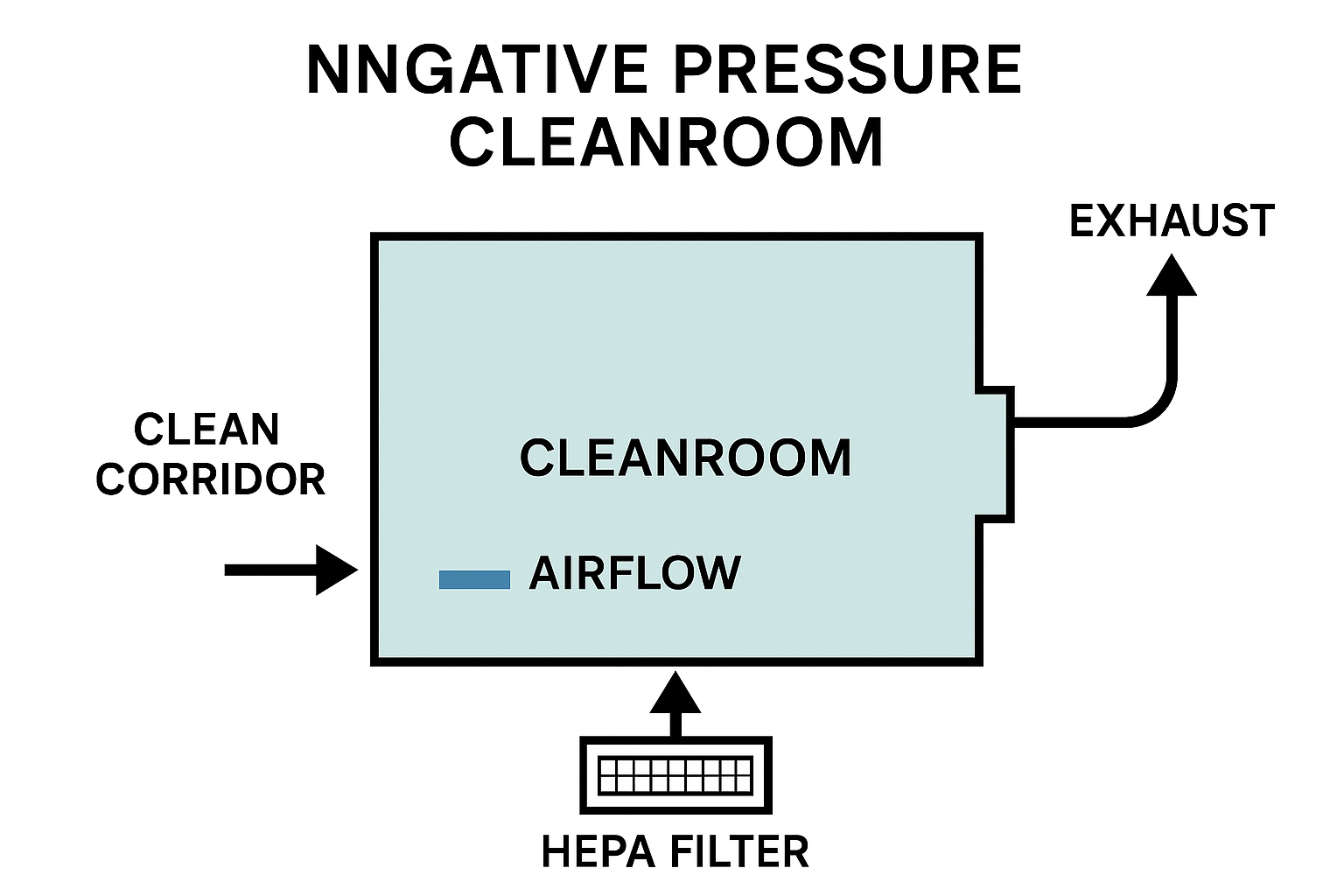
Modular Cleanroom System for Faster Deployment



Stable Temperature, Humidity & Cleanliness Control


Related Cases
Biological Industry Cleanroom——ISO5 70m²
Our biotech cleanroom solutions break through the traditional pharmaceutical cleanliness standards and are designed for cutting-edge fields such as cell and gene therapy, synthetic biology, and microbiome. The use of dynamic pressure gradient control systems and molecular-level air purification technology not only meets the ISO 14644-1 standard, but also achieves precise control of 0.1μm ultrafine particles.Through modular negative pressure isolation units and flexible space layout, we provide biotechnology companies with full-chain environmental protection from GLP laboratories to GMP production.


Pharmaceutical Industry Cleanroom——ISO7 30m²
Cleanroom design includes key control measures such as intelligent personnel/logistics diversion, air lock buffer system, dynamic environmental monitoring, etc., which are suitable for the production and packaging of sterile preparations, biopharmaceuticals, vaccines, injections and highly active drugs. Through full-cycle verification (DQ/IQ/OQ/PQ) and digital environmental monitoring systems, we provide pharmaceutical companies with a safe, reliable and compliant clean production environment, helping to improve drug quality and international market access.

Medical Devices Cleanroom——ISO7 146m²
This project strictly follows ISO 14644 and GMP standards to create a modern clean room that meets the cleanliness level of A/B/C/D. It adopts a high-efficiency HEPA filtration system, precise temperature and humidity control, and laminar airflow organization to ensure that air cleanliness, microbial limits, and environmental parameters continue to meet standards. The clean room design includes key measures such as personnel/logistics separation, strict dressing procedures, and real-time environmental monitoring, and is suitable for the production and packaging of high-demand products such as sterile medical devices, implants, and in vitro diagnostic reagents.
Start Your Cleanroom Project Today
Looking to build or upgrade a Medical Device cleanroom?
"Contact us for a free consultation!"
